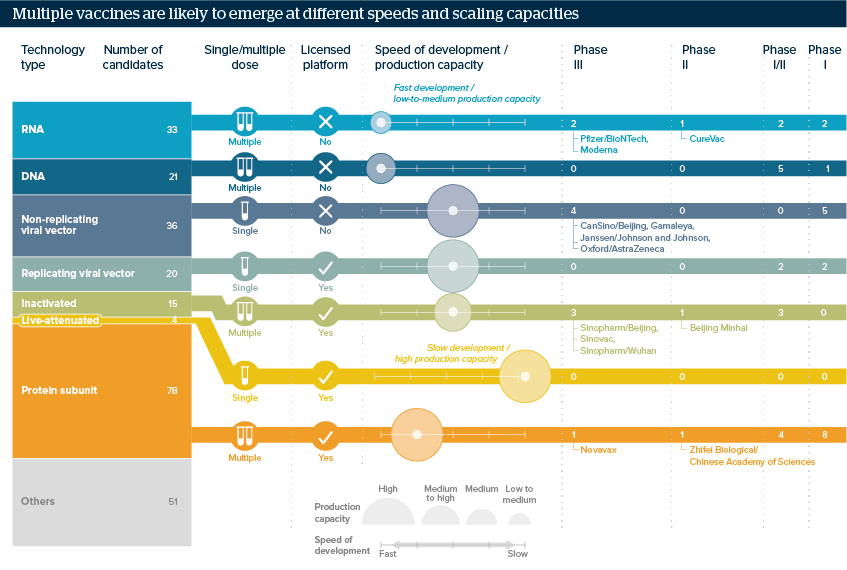
Multiple COVID-19 vaccines will aid distribution
Early vaccines may not be easily scalable, but later ones could be produced faster
Source: LSHTM COVID-19 Vaccine Tracker, WHO, Prof Annelies Wilder-Smith July 2020 presentation LSHTM
Outlook
Over 258 vaccine candidates for COVID-19 are now in development, and ten have reached the last phase of clinical trials before applying for authorisation. Past experience suggests chances of success are around 7% in the pre-clinical phase and rise to 17% once in clinical trials. McKinsey estimates seven to nine vaccines are likely to emerge, and more than 20 in an optimistic scenario.
Early results are expected by end-2020. Some of the front-runner vaccines are faster to develop, but harder to scale up, such as the Pfizer/BioNTech and Moderna RNA candidates. Others, such as live-attenuated vaccines, are harder to develop, but easier to produce at mass scale.
The emergence of multiple vaccines would greatly aid global distribution. The world would be able to take advantage of different production processes and facilities to avoid supply and manufacturing shortages that could occur were there only a single vaccine candidate.
Impacts
- Different vaccines would vary in their effectiveness, including among age groups, and require different roll-out strategies.
- Two-dose injections will require significantly more production, logistical support and awareness campaigning.
- ‘Vaccine hesitancy’ could undermine inoculation campaigns without concerted efforts to address it.
See also
- Variants may delay, not derail COVID-19 control - Feb 15, 2021
- Multiple vaccines enable versatile pandemic response - Jan 26, 2021
- Prospects for COVID-19 in 2021 - Nov 19, 2020
- World should turn corner in COVID-19 fight in 2021 - Nov 17, 2020
- Despite promising vaccines, distancing will stay on - Nov 16, 2020
- Early COVID vaccine hopeful, but more will be needed - Nov 10, 2020
- Global COVID vaccine roll-out will face many hurdles - Oct 14, 2020
- Vaccine nationalism could prolong pandemic - Sep 2, 2020
- More graphic analysis